The industry-funded prospective study found the monoclonal antibody was not associated with adverse outcomes for mother or baby during and after pregnancy.
The first large-scale investigation regarding the safety of vedolizumab in pregnant patients with ulcerative colitis or Crohn’s disease yielded positive results.
There is often little to no human safety data for pharmaceutical products in pregnant women – and using vedolizumab to treat pregnant patients with Crohn’s disease or ulcerative colitis is no exception.
To overcome this knowledge gap, researchers from the University of California San Diego have conducted an observational registry study to add novel, prospective data relating to the safety of vedolizumab for CD or UC when used in pregnancy.
“We found no meaningful evidence of increased risks for major structural birth defects or any of the other adverse outcomes under study,” the researchers wrote in the American Journal of Gastroenterology.
“While the sample size was limited, and the study relied on volunteer participants, the findings of this study were consistent with three other previously published controlled studies. Taken together, the available evidence does not suggest an increased risk for any of the adverse pregnancy or infant outcomes studied.”
Researchers recruited 275 pregnant women who fell into one of three categories: 95 women with CD or UC who used vedolizumab during pregnancy, 76 women with CD or UC who did not use vedolizumab and 100 women without chronic disease or vedolizumab use.
All women completed as many as three telephone interviews over the course of their pregnancy, and an additional interview post-pregnancy, where demographic, medical and behavioural data were obtained. Other birth- and child-related information were collected from medical records as needed.
Related
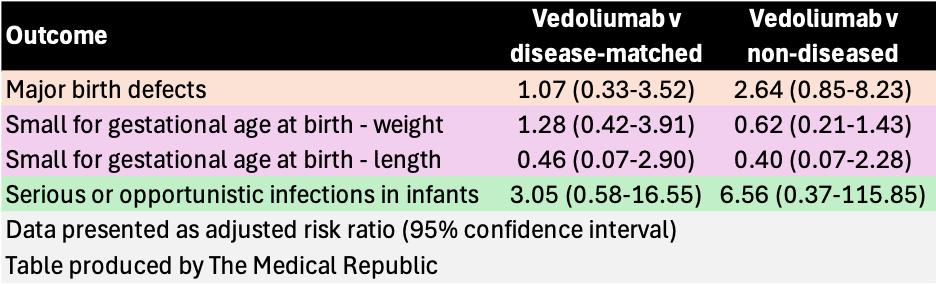
There was no difference on any of the collected outcomes between the vedolizumab-exposed group, the disease-matched group and the non-diseased, unexposed group, including spontaneous abortion, stillbirth, preterm delivery and the developmental outcomes presented in the table below.
Vedolizumab is a humanised monoclonal antibody designed to target lymphocyte integrins, a family of receptors on the cellular surface that influences T cell migration, activation and function. The treatment prevents leukocytes from migrating into gastrointestinal mucosa and is therefore used as a treatment in Crohn’s disease and ulcerative colitis.
The study was funded by the Takeda Pharmaceutical Company, which developed vedolizumab. Although the researchers claimed responsibility of the contents of the manuscript, Takeda had a level of input into the research and publication process.
“As this study was conducted to meet a post-marketing regulatory responsibility, the funder provided input on the study design and the plan for analysis and had the opportunity to provide comments on the manuscript,” the researchers disclosed.





