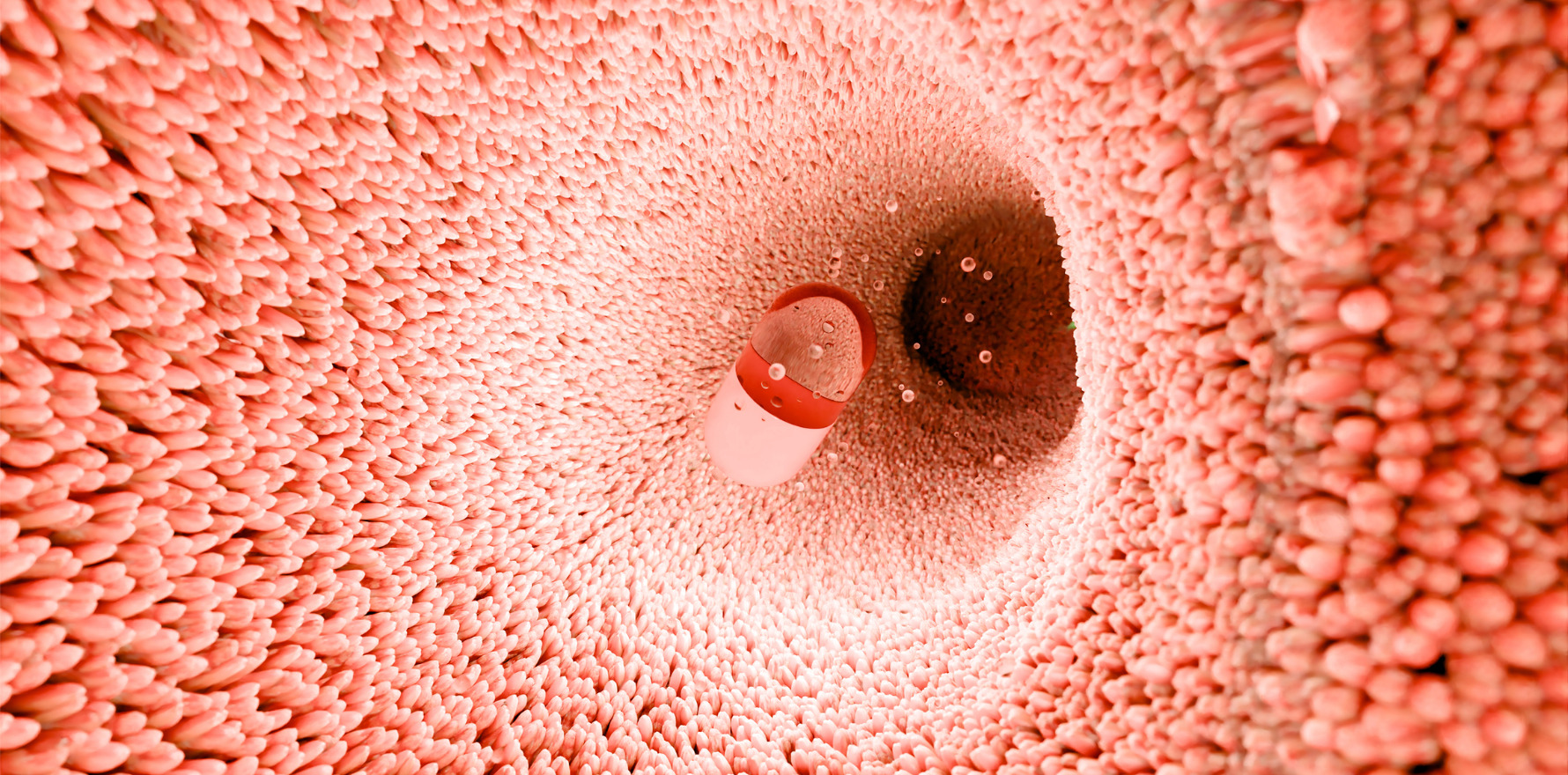
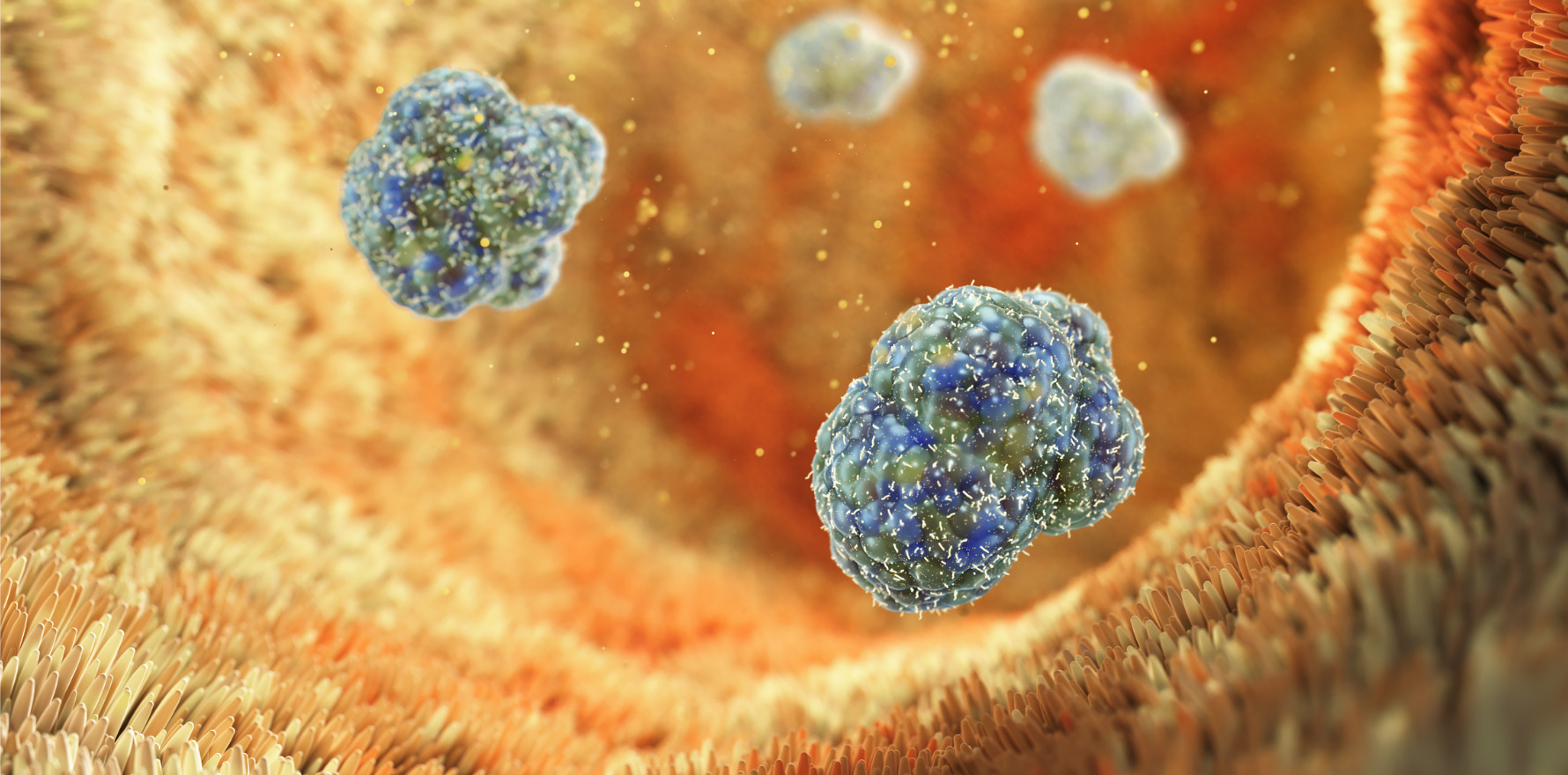
Ingestible device triggers engineered E. coli to fight colitis in pigs, paving the way for precision microbiome therapies.
Researchers have created a smartphone-controlled ingestible capsule that can monitor and control engineered gut bacteria inside the body.
The development could revolutionise microbiome-based therapies for inflammatory bowel disease (IBD) and other gastrointestinal disorders, they say.
As proof of concept, the researchers induced colitis in three pigs and colonised them with modified E. coli that emitted light when detecting nitrate, a marker of inflammation. The capsule picked up this signal and transmitted it to the app, alerting researchers.
In response, they used the app to flash the capsule’s LED, activating a light-sensitive genetic circuit in the bacteria that prompted secretion of anti-inflammatory antibodies.
The approach successfully alleviated colitis symptoms in the animals.
Published in Nature Microbiology last month, the study combines synthetic biology, optoelectronics, and wireless communication to establish a bidirectional biological–optical–electronic signal processing chain, enabling direct interaction with engineered microbes in vivo.
Engineered bacteria, such as Escherichia coli, have shown promise for detecting biomarkers and delivering therapeutic molecules to treat IBD, cancer, and metabolic conditions. However, once inside the gut, their activity cannot currently be tracked or adjusted, presenting a major barrier to clinical translation.
Chemical inducers have been tested to regulate microbial circuits, but these diffuse unpredictably and degrade inside the body. Light-based control offers better precision, but delivering optical signals to deep gut regions without invasive tools has remained a challenge.
The research team integrated optogenetically engineered microbes with an ingestible optoelectronic capsule, controlled through a wireless smartphone interface.
The team modified E. coli Nissle 1917 to sense nitric oxide (NO), a marker of intestinal inflammation, and emit a bioluminescent signal for detection. A second genetic circuit was added to respond to light by secreting Nb-TNF, an anti-inflammatory nanobody targeting tumour necrosis factor (TNF).
About the size of a large pill, the capsule contains a light sensor, LED, and custom-printed circuit board powered by button batteries. It detects microbial luminescence, converts it into an electrical signal, and relays this data to a smartphone app.
The app then allows clinicians to send commands back, prompting the capsule’s LED to flash and activate bacterial therapeutic release.
The wireless connection to a smartphone interface provides clinicians (or in future, patients) with real-time feedback and control over bacterial behaviour inside the gut.
The team tested the system in the pigs with induced colitis. Engineered E. coli detected nitric oxide and emitted a luminescent signal, which the capsule captured and transmitted to the smartphone app.
Researchers then activated the capsule’s LED remotely, triggering the bacteria to produce and release the anti-inflammatory nanobody, significantly reducing inflammation.
Extensive in vitro, ex vivo, and in vivo studies validated the platform’s ability to sense faint luminescence signals and deliver controlled optogenetic activation, they wrote in the article.
Related
The researchers said potential future applications include IBD management, cancer therapy, metabolic regulation, and daily health monitoring.
“The next-stage aim of our strategy is to build up a closed-loop microbial-optoelectronic signal processing cycle to assist daily health supervision,” they wrote.
“This aim clearly defines the monitoring and regulation process of user participation, different from the previously reported strategies working in a biomarker-triggered self-tunable treatment manner.
“The disease-related markers and therapeutic targets can be expanded to physicochemical properties, microbiome changes and biological macromolecules.”
They said that recently “remarkable” studies had expanded the types of detected marker and the diversity of treatment modes using engineered microbes.
“In addition, the updated technologies of probiotic delivery, conditional colonisation and microbial community engineering will enable long-term daily health administration,” the authors concluded.