Some unregistered products contained over four times the stated dose, others none at all.
Australia’s medicines watchdog has issued a safety advisory regarding imported unregistered melatonin products following testing by TGA Laboratories.
The results revealed significant discrepancies between the labelled and actual melatonin content, with one product containing more than 400% of the stated amount and another containing no detectable melatonin.
The products tested by TGA Laboratories are known unregistered therapeutic goods. These products can often be purchased online or from local retailers. However, they may not meet Australian standards for safety, quality and effectiveness, the regulator said.
“This variability in melatonin content raises serious safety concerns for consumers, including the risk of hospitalisation and accidental overdose, especially in children,” the TGA said in a statement.
The report revealed products found to contain significantly more than the labelled amounts included Spring Valley Melatonin 10mg (variation from labelled amount – 119%-136%); Natrol Melatonin 5mg gummies (140%-170%); CVS Health Melatonin 3mg (112%-121%); The Smurfs Kids Gummies Melatonin 1mg (155%-170%); Natrol Advanced Sleep Melatonin 10mg (112%-123%) Sleepose-3 Melatonin 3mg (209%-417%); Nutraceutical Sleepose-3 Melatonin 3 mg (95%-174%) Vitafusion Sleep Well – Melatonin 3mg (106%-124%).
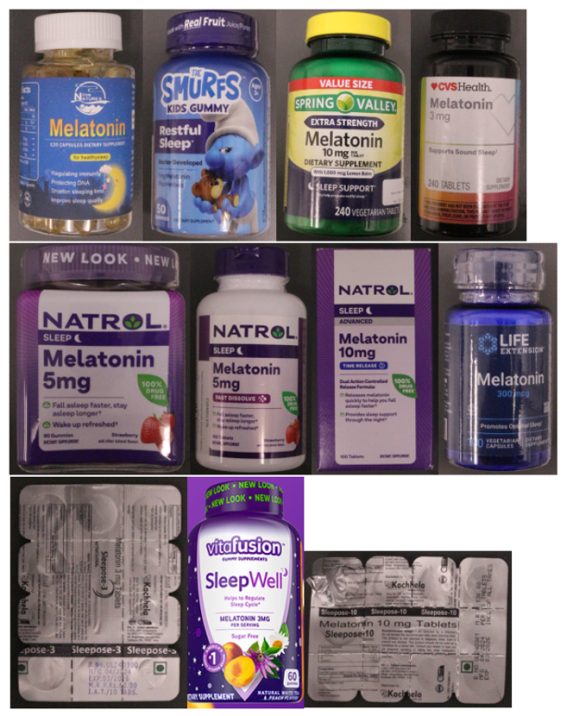
Products found to contain significantly less than the labelled amounts included Life Extension Melatonin 300mcg (variation from labelled amount – 79%-97%); Sleepose-10 Melatonin 10mg (37%-59%); and Live Natures Melatonin 10mg (no melatonin detected).
“Consumers are advised that melatonin is a prescription-only medicine in Australia, except in specific circumstances for adults,” said the TGA.
“Parents are strongly encouraged to consult their child’s treating medical practitioner regarding the use of melatonin, especially for children with seizure disorders as it may increase seizure frequency.
“Melatonin products taken without appropriate medical oversight can cause serious side effects including hospitalisation and accidental overdose, especially in children.”
The tested products are unregistered therapeutic goods that may not meet Australian standards for safety, quality, or efficacy, the TGA warned.
Health professionals should advise patients to check for an AUST R or AUST L number on the packaging, which confirmed the product contained accurate ingredients and was manufactured under strict safety and quality standards.
Patients should be instructed to stop using unregistered melatonin products immediately and take any remaining items to a local pharmacy for safe disposal. They should also seek medical advice if any adverse effects occur and report suspected side effects to the TGA.
In addition to its safety advisory, the TGA issued a statement about the regulation of melatonin products in Australia.
“There has been an increase in Australians purchasing melatonin products from online stores, particularly for use in children. These products are often sold as gummies but are also available as tablets or capsules marketed as dietary supplements,” it said.
“Consumers who import these unregistered ‘melatonin’ products from online stores for personal use or use by an immediate family member risk serious health problems.
“In Australia, melatonin is only approved for use in children as a prescription-only medicine in limited circumstances.”
Specifically, melatonin is approved for the treatment of insomnia in children and adolescents (2-18 years) with autism spectrum disorder (ASD) and/or Smith-Magenis syndrome.
“The TGA has not evaluated the safety or efficacy for broader use in children,” it said.
“The TGA advises that children suffering from any medical condition, including sleep disorders or irregular sleep patterns, should see a doctor and not be given medicines purchased over the internet.”
The TGA said it would continue to “monitor signals relating to harmful unregistered products and will notify the Australian Border Force (ABF) to seize and destroy any of these products intercepted at the border”.


